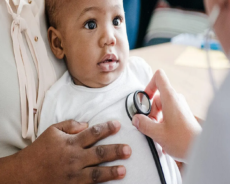
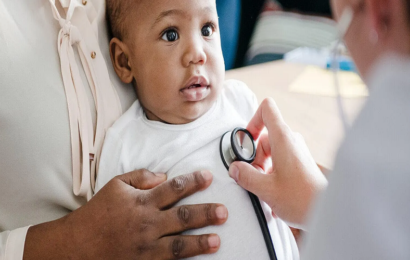
A crystalline form of NicotinamideRiboside chloride termed NIAGEN has been evaluated in a battery of preclinical studies including a bacterial reverse mutagenesis assay, an in vitro chromosome aberration assay, an in vivo micronucleus assay, and acute, 14-day and 90-day rat toxicology. In the 90-day toxicology study, NR had a similar toxicity profile to NAM at equimolar doses, the lowest observed adverse effect level for NR was 1000 mg/kg/day, and the observed adverse effect level (NOAEL) was 300 mg/kg/day. NIAGEN is Generally Recognized as Safe (GRAS) in the United States for use in food products and the subject of two new dietary ingredient notifications, which were filed with the United States Food and Drug Administration without objection. Discover more about Nicotinamide Riboside Chloridein this article.
To date, NR has also been tested in six clinical trials.
First clinical trial:
NR established the safe oral availability of single doses and the time course by which NR elevates the human blood NAD metabolome.
Second clinical trial:
This trial provided additional safety data for healthy people taking NR for 8 days.
Third and fourth clinical trials:
NR safety in healthy people was addressed either by taking 500 mg NR twice daily for 6 weeks or combination of up to 500 mg NR and 100 mg pterostilbene per day for 8 weeks. Whereas, it was found that the combination of NR and pterostilbene significantly elevated low density protein cholesterol in a dose and time-depended fashion, no significant increases in LDL-C were seen following the administration of NR alone.
Fifth clinical trial:
A fifth clinical trial documented the safety and tolerance of ingesting 2 grams NR per day for 12 weeks in obese men and post hoc analyses suggested that there was an improvement in fatty liver in the NR-treated group.
Sixth clinical trial:
Single 500 mg doses of NR depressed markers of oxidative damage while increasing NADPH and exercise performance in older individuals.
Nicotinamide is a pyridinecarboxamide that is pyridine in which the hydrogen at position 3 is replaced by a carboxamide group. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor, a metabolite, a cofactor, an antioxidant, a neuroprotective agent, an EC 3.5.1.98 (histone deacetylase) inhibitor, an anti-inflammatory agent, a Sir2 inhibitor, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a pyridinecarboxamide and a pyridine alkaloid. It derives from a nicotinic acid.
It is classified under the vitamins and is generally used as a nutritive supplement for Vitamin B. Nicotinamide, also called Niacinamide is present in all living cells and it is essential for normal growth and health. Nicotinamide is used as a dietary supplement and medication. It is also used in personal care products like Magnesium L-Threonate Supplements.
EXPOSURE: Workers who use Nicotinamide may breathe in vapors or have direct skin contact. The general population may be exposed by consumption of food and administration of medications. If Nicotinamide is released to the environment, it will be broken down in air. It is also expected to be broken down by sunlight. Nicotinamide will not move into air from moist soil and water surfaces. It is expected to move through soil and will be broken down by microorganisms, and is not expected to build up in fish.